Why Partner?
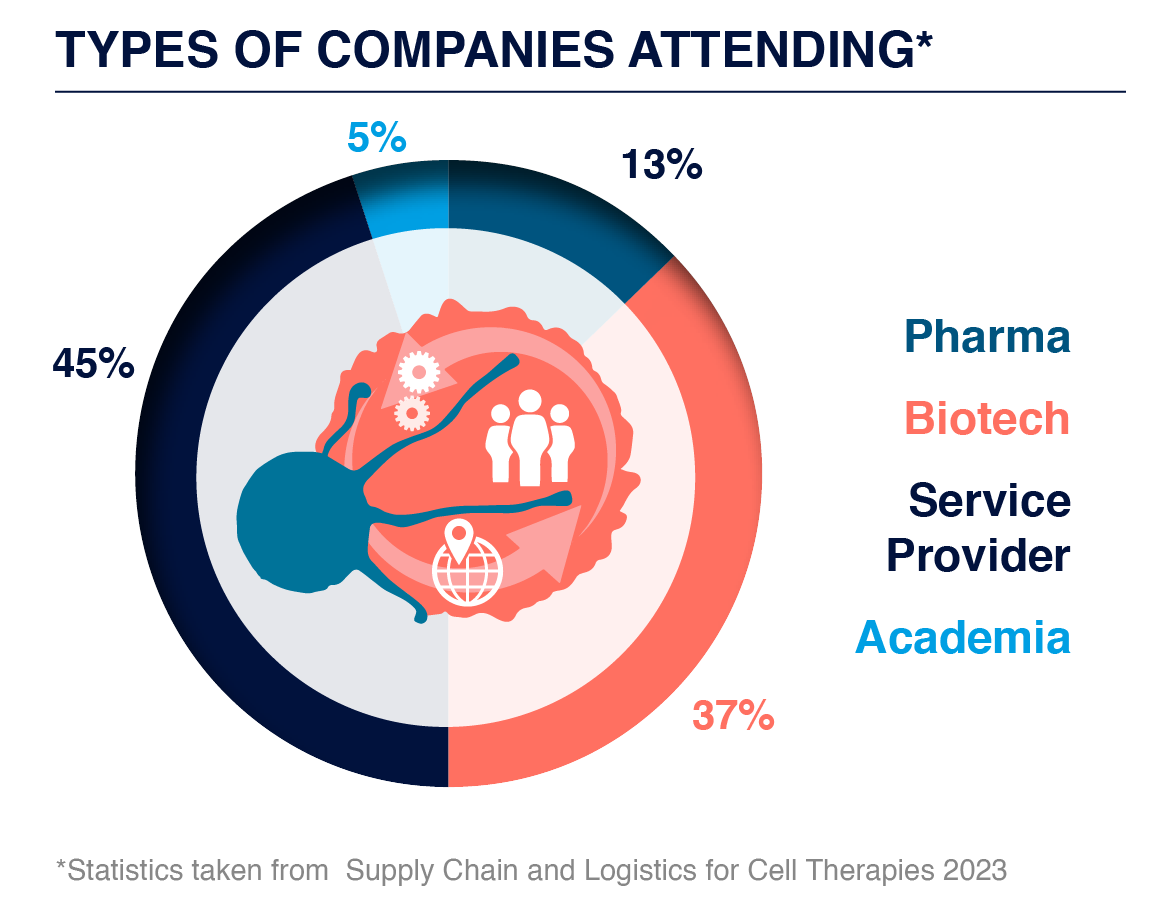
Attending Companies Included:
As we kicked off 2024, there were 2109 CAR and TCR active clinical trials, but as these trials progressed, there were key bottlenecks limiting their scalability. Drug developers are actively seeking support in digitalization, regulations consultancy, cold-chain solutions, blood banks and logistics management to enable phase-appropriate and cost-effective expansion.
3 Reasons the 5th Supply Chain & Logistics for Cell Therapies Summit Was a Priority Event This Year:
1.
It was the only meeting dedicated to addressing the specific challenges associated with cell therapies, and therefore provided a perfect opportunity to showcase your thought leadership with the decision makers across biotech, pharma and clinical sites.
2.
Those working in supply chain and clinical operations are actively seeking out new and cost-effective partners to support and streamline their processes, therefore the agenda was developed to host dedicated 1-to-1 networking, small group discussion sessions and plenty of panels to meet new leads and showcase expertise.
3.
Uniting 100 decision makers across cell therapy teams, this intimate forum provided an opportunity where everyone in the room was relevant, and every session was valuable in navigating shared challenges – meaning the quality of leads was unmatched.
What You Could Gain:
Differentiate your product from competitor logistic couriers, digital orchestrators or consultant service providers to become the industry’s partner of choice.
Showcase your capabilities and position your service or product to the leading experts at each touchpoint of the cell therapy logistics, from transport to manufacturing to collection to patient logistics.
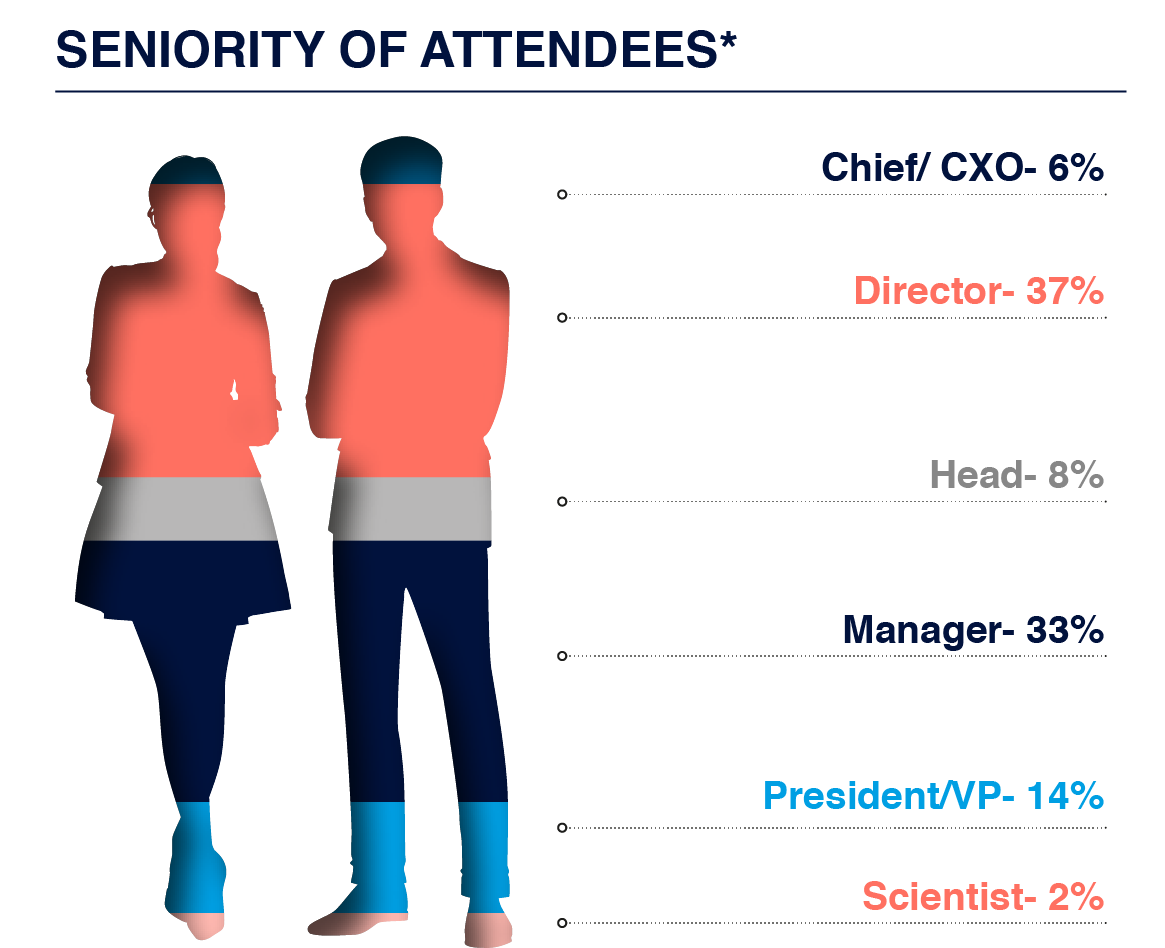
Build your network with Directors, Heads and Vice Presidents from across biotech, pharma and hospitals to form long-lasting business relationships.
Who You Could Have Met?